-

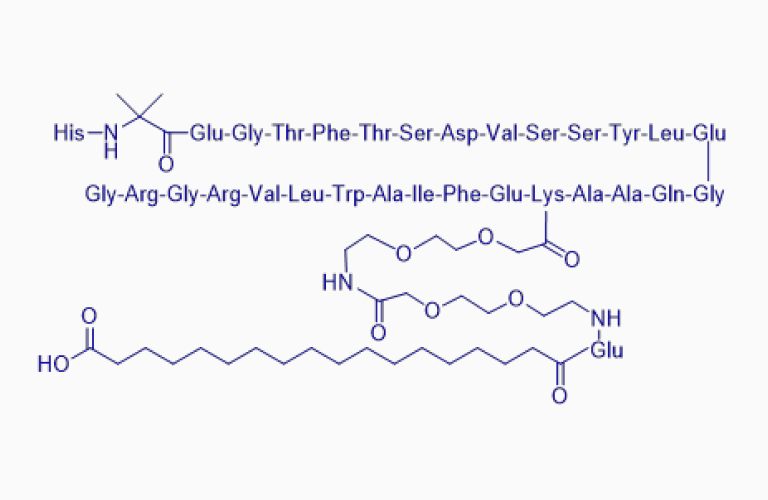 Semaglutide*
Semaglutide*CAS:910463-68-2
Certification:USDMF
-

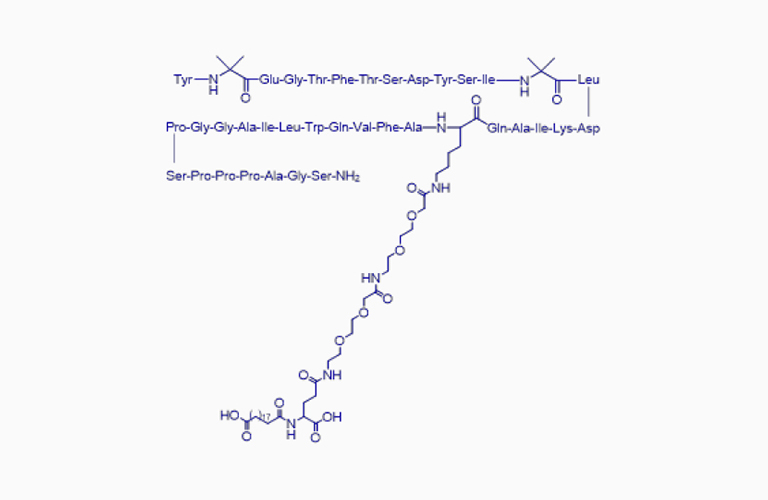 Tirzepatide*
Tirzepatide*CAS:2023788-19-2
-

 Liraglutide*
Liraglutide*CAS:204656-20-2
Certification:USDMF
-

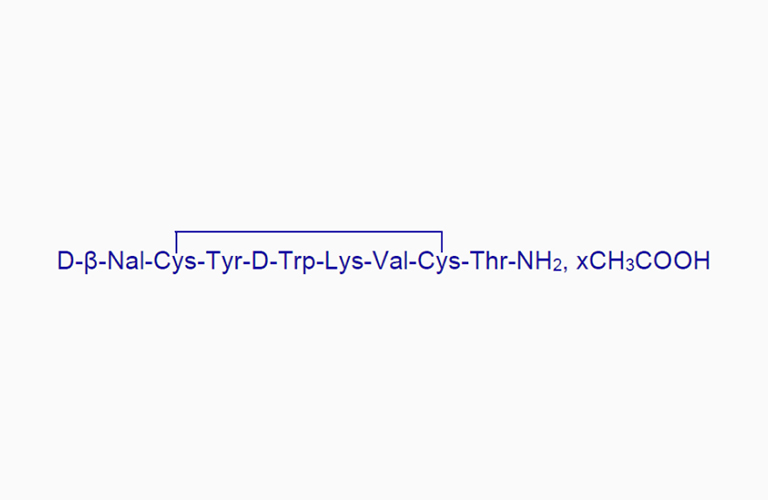 Lanreotide Acetate
Lanreotide AcetateCAS:127984-74-1
-

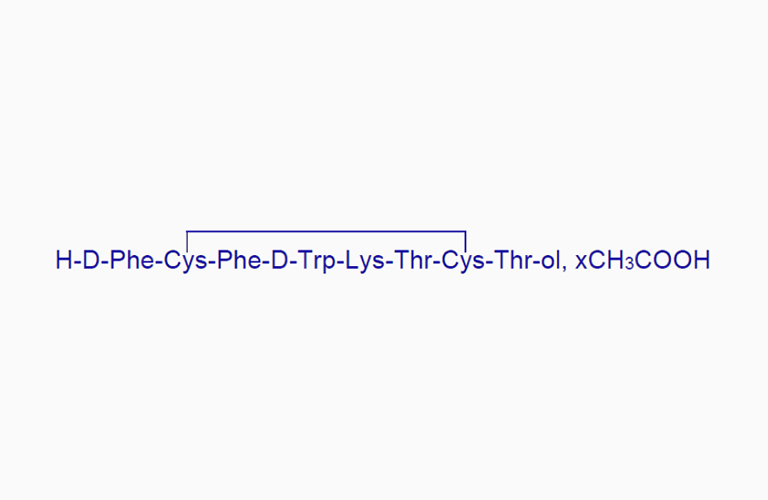 Octreotide Acetate
Octreotide AcetateCAS:79517-01-4
-

 Cetrorelix Acetate
Cetrorelix AcetateCAS:145672-81-7
-

 Desmopressin Acetate
Desmopressin AcetateCAS:62357-86-2
-

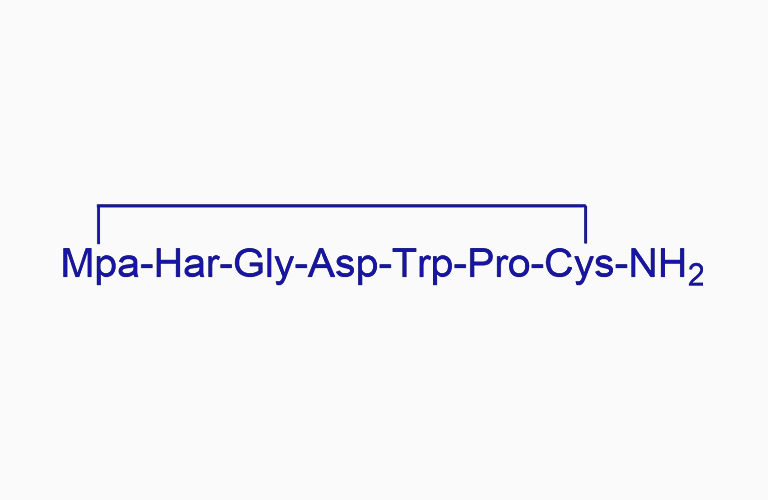 Eptifibatide
EptifibatideCAS:148031-34-9
-

 Bivalirudin
BivalirudinCAS:128270-60-0
-

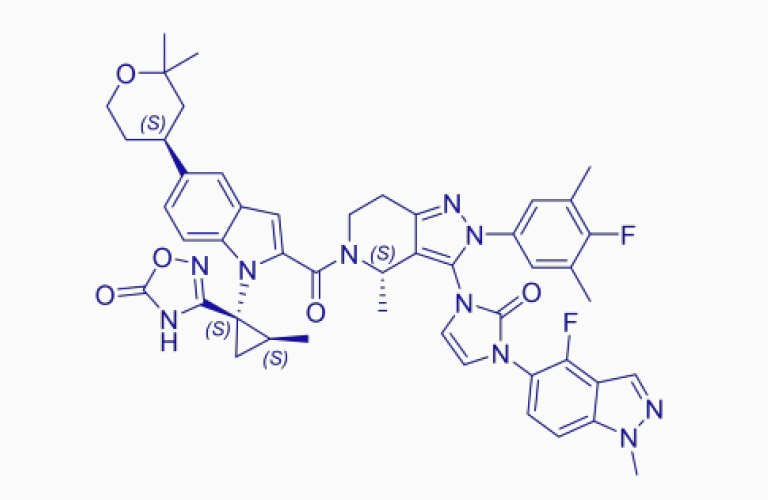 Orforglipron*
Orforglipron*CAS:2212020-52-3
-

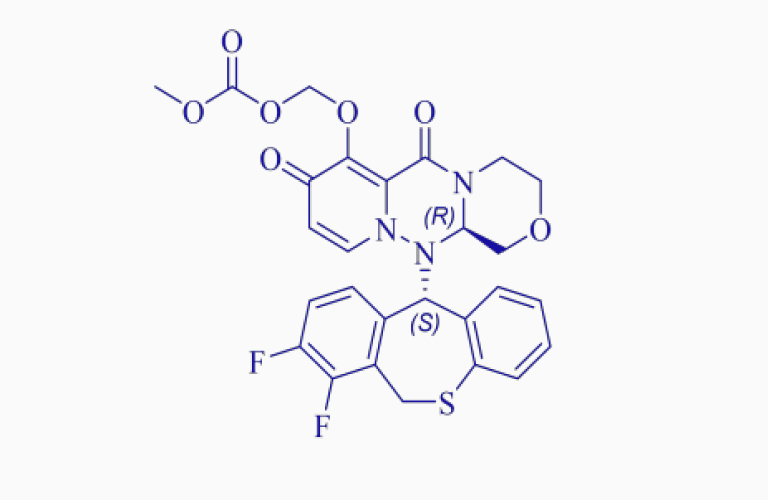 Baloxavir marboxil*
Baloxavir marboxil*CAS:1985606-14-1
-

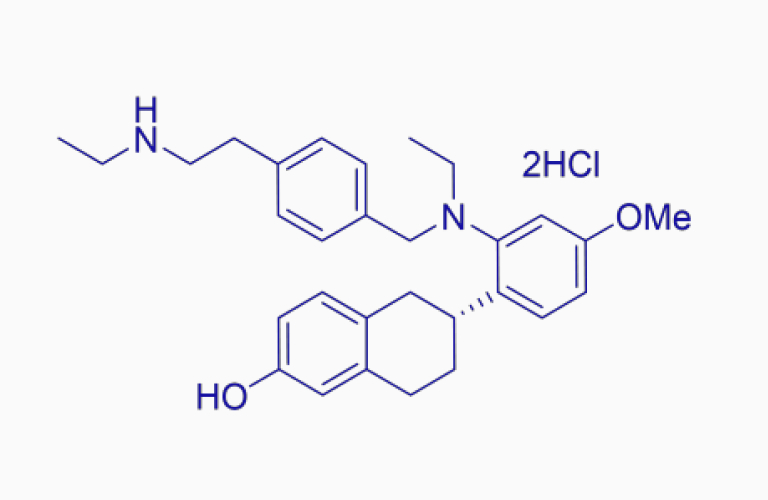 Elacestrant dihydrochloride*
Elacestrant dihydrochloride*CAS:1349723-93-8
Product Pipeline
-
R&D
-
Pilot
-
Validation
-
DMF file
-
Commercial
-
Peptides
-
Small Molecules
-
Oligonucleotides
-
Bivalirudin
-
Cetrorelix Acetate
-
Eptifibatide
-
Lanreotide Acetate
-
Octreotide Acetate
-
Thymalfasin
-
Atosiban Acetate
-
Desmopressin Acetate
-
Liraglutide*
-
Semaglutide*
-
Tirzepatide*
-
Edotreotide
-
Oxytocin
-
Retatrutide*
-
Teriparatide
-
Difelikefalin*
-
Pegcetacoplan*
-
Plecanatide
-
Setmelanotide*
-
Teduglutide
-
Ziconotide
-
Agomelatine
-
Atorvastatin Calcium
-
Epalrestat
-
Fulvestrant
-
Oseltamivir Phosphate
-
Sodium Picosulfate
-
Alogliptin Benzoate*
-
Roxadustat*
-
Sulindac
-
Ezetimibe
-
Risdiplam*
-
Baloxavir marboxil*
-
Elacestrant dihydrochloride*
-
Dydrogesterone
-
Elacestrant*
-
Orforglipron*
-
Rimegepant*
-
Ubrogepant*
-
Nusinersen*
-
CPG 1018
-
CPG 2007
-
CPG 2216
-
Givosiran*
-
Inclisiran*
-
Patisiran*
-
Vutrisiran*
*Disclaimer: Due to the limitation of patent protection, commercial production and sales will not be carried out for the time being, and will only be used for scientific research and administrative approval.










